Abstract
Introduction: Overall response rate (ORR), defined as complete or partial response as per 2014 NIH consensus criteria (Lee et al, BBMT 2015), is commonly used as key endpoint to assess treatment efficacy of chronic graft versus host disease (cGvHD), either as ORR at week 24 or as best overall response rate (BOR) at any time point up to and including week 24. Both endpoints as well as duration of response (DOR) were previously reported in the REACH-3 study, a phase 3 open-label, randomized study comparing ruxolitinib (RUX) versus best available therapy (BAT) for glucocorticoid-refractory or dependent (SR/D) chronic graft-versus-host disease. RUX demonstrated superior efficacy compared with BAT, with a higher ORR at week 24, higher BOR at any time up to week 24 and longer DOR (Zeiser et al. NEJM 2021). The comparison between RUX and BAT was performed on ORR and BOR using all randomized patients, while DOR was derived for the subgroup of responders only. To further assess the "breadth and depth” of clinical benefit in cGvHD, a novel measure of efficacy is presented in this post-hoc analysis.
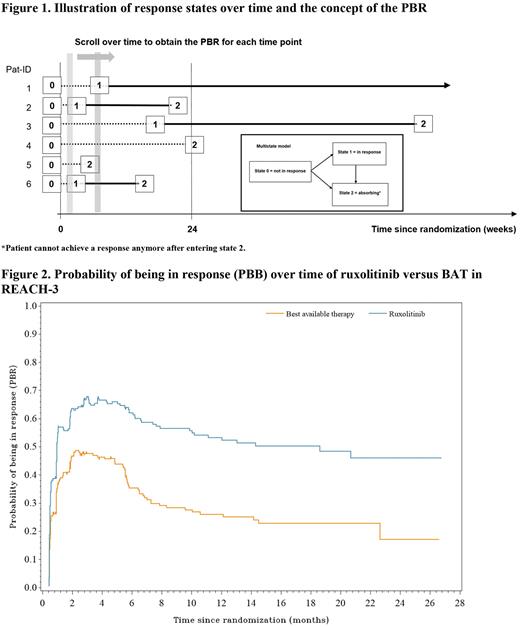
Material and methods Here we illustrate the application of the probability of being in response (PBR), a graphical method presenting simultaneously the time to first response and subsequent failure, i.e. combining time to first response, response rates and DOR into one measure. Considering the PBR as a function of time was suggested by Temkin (Biometrics 1978). As illustrated for six hypothetical patients (Figure 1), all patients are in state 0 (not in response) at baseline (=study start). A patient responding to treatment enters state 1 = in response at the time of the first documented response. Such patients may lose their response at a later time point and enter the absorbing state 2 (Pat-ID 2, 3 and 6, Figure 2) or are still in response at the time of the statistical analysis (Pat-ID 1), in which case they are censored in state 1. Patients who die or progress or change type of systemic cGvHD treatment or do not achieve response within 24 weeks switch from state 0 to state 2 (Pat-ID 4 and 5, Figure 2). For example, Pat-ID 5 might have died (absorbing event) without response and Pat-ID 4, who did not respond up to week 24 (absorbing event) may, then have changed systemic cGvHD treatment (also an absorbing event). Any patient who did neither reach state 1 nor state 2 (i.e. drop out before week 24 without any of the events defined as state 2) would have been censored in state 0, in REACH-3 this happened for a few patients only. PBR is estimated by applying time-to-event methodology similar to the well-known Kaplan-Meier plot.
Results: The median time to first response for all randomized patients (with non-responders censored) was 29 days (95% CI: 24 to 31 days) for patients receiving RUX and 50 days (95% CI: 29 to 57 days) for patients receiving BAT. The plot of the PBRF (Figure 2) shows a consistently higher probability of being in response at all time points. The larger area under the curve in the ruxolitinib arm also shows that a longer average duration of response is achieved in this arm than in the BAT arm.
Conclusions: In REACH-3 ruxolitinib patients had a higher probability of being in response at all time points as measured by the PBR function, a novel efficacy endpoint that allows a valid comparison between randomized treatment arms and depicts long term benefit combining response and its duration visualizing all patients, not only responders. PBR may be a useful measure that could be added to future updates of cGvHD response criteria to better describe the benefits of cGvHD therapy.
Disclosures
Hollaender:Novartis: Current Employment. Glimm:Novartis: Current Employment, Current equity holder in publicly-traded company. Gauvin:Novartis: Current Employment, Current equity holder in publicly-traded company. Stefanelli:Novartis: Current Employment. Zeiser:Novartis Pharmaceuticals: Honoraria, Speakers Bureau; Mallinckrodt: Honoraria, Speakers Bureau; Incyte Corporation: Honoraria, Speakers Bureau.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal